Pictured above is the E. coli bacteria. Some strains of these are pathogenic, others (found in your digestive system) aren't dangerous at all.
Immunoglobulin structure, function and diversity
There are five classes of immunoglobulin (antibody) molecules found in your body's arsenal - they all share a common basic structure which can be seen in other mammals' antibodies too. Every antibody is consists of one to five different immunoglobulins; when there is more than one immuoglobulin present, they are linked together by a molecule called a J chain. Antibodies are large molecules that we call a polymer as it is made of smaller units called monomers, in this case they are in the form of immunoglobulin - joining together to make a larger antibody unit*.
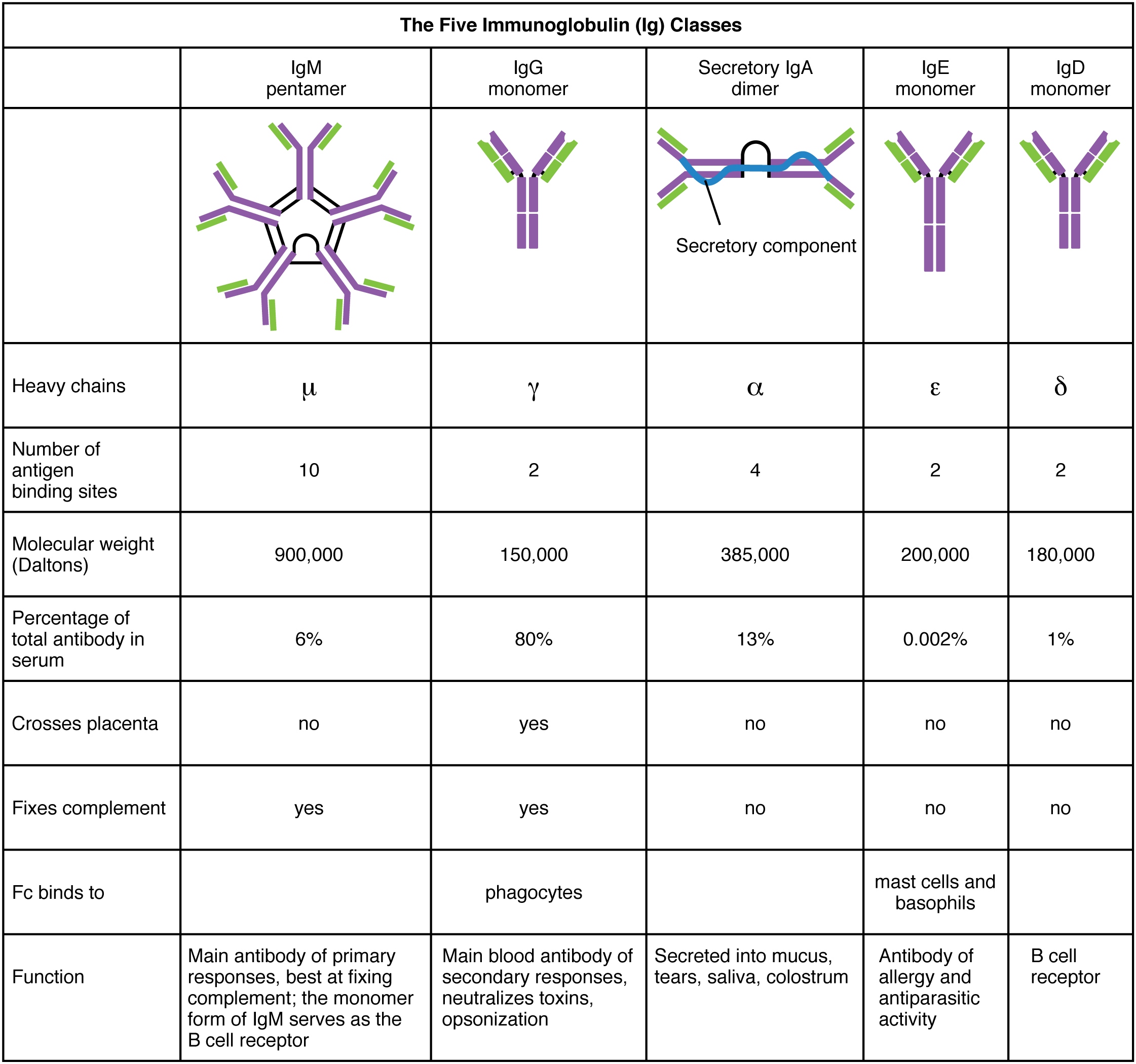
*To clarify - in the previous section I said immunoglobulin and antibody are interchangeable, this is still true as individual immunoglobulin molecules join together (as each has a different function) and form what we call an antibody. Being made of several differnt immunoglobulin monomers gives the antibody versatility when engaging threats. The different types of a immunoglobulin monomers are below:
The immunoglobulin monomer is made of four polypeptide chains and can be separated into two forms: two heavy chains with a molecular weight of 53,000 Da (Daltons) and two light chains with a molecular weight of 23,000 Da each. Each of these chains are held together by Disulfide bonds (S-S) and contain regions of called domains. These domains are either constant (the same in any antibody of a type of class) or variable.
The variable domains of these chains determine how the antibody binds to a specific antigen (foreign object). Large objects, such as a protein, virus or bacterial cell have different parts that can be recognised by an antibody (antigenic determinants), these areas are usually scattered on the object's surface, allowing more than one antibody to bind and force the antigen to aggregate. This type of aggregation is called immunoprecipitation - and works by isolating a threat to be destroyed that contains thousands of different proteins or antigenic determinants. Immoprecipitation is a process that requires an antibody to have a bivalent structure, meaning it needs two areas in which it can bind to the antigen.
In the lab, it's possible to break up the antibody into fragments ("cleavage"); the moleucle is of say, IgG is Y shaped and produces three fragments: two Fabfragments contains a binding site each) and a single Fc fragment (contains no binding sites for antigens). See the image below, noting that V denotes a variable chain and C denotes a constant chain. It's also important to note that the innermost chains of the Y shape are heavy chains while the outermost chains are the light chains:

The constant domains (C) help keep chains together and also act as signalling regions (effectors) to other cells involved in immune responses such as T-cells or macrophages (white blood cells).
The artful nature of antibodies: the immunoglobulin superfamily
Domain areas in immunoglobulins have a common canvas called the Ig domain which most likely presents a primitive structural element found in the evolution of the immune response. Proteins that have this Ig domain are classed as being part of the immuoglobulin superfamily. This domain is a very stable scaffold which hold the hypervariable molecular loops which determine the shape and charge of the areas that bind to antigens. The molecular loops are called complementary determining regions (CDRs).
The CDRs are what determines whether or not the antibody will bind to an antigen, this is due to the shape and charge complementarity of the CDRs. Shape complementarity occurs because the three-dimensional shape of the antibody binding area and the shape of the antigen complement each other and fit like a puzzle piece or say, a glove. Charge complementarity on the other hand occurs when weak interactions between the target antigen and antibody, such as van der Waals, hydrogen bonding and electrostatic attractions. These interactions are the same as explained in my other blog here. These types of interactions are useful in biochemistry as they help explain many different types of biochemical processes, such as the structure of DNA.
It is our understanding of the molecular binding between the CDRs and the antigens that helps efforts to create vaccines against the deadly hepatitis B - affecting around 400 million people worldwide.
Generating Antibody diversity
Throughout life's time here on earth, particularly in large animals such as ourselves and our ancestors, B-cells that produce antibodies have undergone many mutation events such as sequence rearrangement or splicing that have resulted in numerous combinations of genetic code. The genetic code for IgG immunoglobulin (in the table) CDR loops has historically mutated at an unusually high rate in mammals and accounts for the diversity of combinations of IgG immunoglobulins found in the human genome (there's around 10 billion combinations!). The mutation events are random and are therefore not pre-programmed thus it is possible for a white blood cell (B lymphocyte) to produce an immune response to synthetic substances that have a binding sites complementary to the antibodies.
The next section will cover some cellular immune response cells involved in producing antibodies such as killer T cells and why it's so hard to develop a vaccine for AIDS.
Thanks for reading!

No comments:
Post a Comment