What is Molecular Orbital (MO) theory?
Molecular orbital theory is an expansion on VSEPR theory (check the link if you need a referesher), which you should've learned about in High School. MO theory differs from VSEPR theory, in that it takes into account regions of space spread over the entire molecule in which you might find an electron. MO theory allows us to calculate these areas, called molecular orbitals, the nuclei of the atoms are placed in their equilibrium position for these calculations. Molecular orbitals form from interactions arising in the molecule, between the nuclei and the orbitals surrounding them. These interactions are restricted by several factors:- Interactions are allowed only if the symmetries of the atomic orbitals are compatible, i.e. they can 'fit' together;
- The relative energies of the atomic orbitals is also very important, as the further apart in terms of energy each atomic orbital is, the less likely they will be to form a molecular orbital;
- the region of overlap between atomic orbitals needs to be significant enough for a MO to form.
So what if we apply this so a very simple molecule, such as H2?
MO Theory in H2

Molecular orbitals are indicated by
wavefunctions, 𝜓, which describe the nature of electrons within that
orbital. Since electrons are also considered waves, the overall sign of
the wavefunction can either be + or -. Additionally, just like waves,
they can be described as transverse waves, i.e. they can interferre in a
constructive (in-phase), described as "bonding" and destructive (out of
phase), described as "anti-bonding" in MOs. We can describe these types
of bonding orbitals as linear combinations of their respective atomic
orbitals. The respective mathematical functions of these orbitals can be
simplified as 𝜓MO(in-phase) and 𝜓*MO(out-of-phase), where N is a
normalizing constant:
𝜓MO(in-phase) = 𝜓MO = N[𝜓1 + 𝜓2]
𝜓MO(out-of-phase) = 𝜓*MO = N[𝜓1 - 𝜓2]
N can be determined the equation below, whee S is the "overlap integral", a measure of how the regions of space:
N = 1 / √2(1+S) ≈ 1/√2
N* = 1 / √2(1-S) ≈ 1/√2
S is not taken into account in the approximate values given. The diagram above shows that the bonding MO, 𝜓MO,
is stabilized by the Is orbitals from the contributing H atoms, to form
the bonding orbital. Conversely, the anti-bonding MO is destabilized,
because the difference in energy between the anti-bonding MO and the 1s
orbitals is slightly bigger than that between the bonding MO - this
effect is far to complicated for me to explain in my blogs, it is
however important to remember.We can represent the anti-bonding and bonding orbitals in 3 dimensions, as anti-bonding orbitals form nodes due to destructive interference; the first orbital shown is the highest occupied molecular orbital, or HOMO for short, and the second is the lowest unoccupied molecular orbital, or LUMO. The HOMO is the bonding orbital as and the LUMO is the anti-bonding orbital:


You would notice that there is separate colours in the LUMO, indicating that there is a node in between and the 1s orbitals that make up the MO are out of phase.
Bonding in other X2 type molecules
Now let's have a look at other molecules, say F2 and O2, which have 2s and 2p orbitals; recall from VSEPR theory that these orbitals have a maximum of 2 and 6 electrons each, respectively. The bond order can be calculated like this:
Bond Order = 1/2(total bonding electrons - total anti-bonding electrons)
We are only concerned with the valence shells, so we'll only display
it as the 2s and 2p orbitals. The number of MOs equals the amount of
atomic orbitals (as in VESPR), from this we can determine that the HOMO
is 1/2 number of total valence electrons, LUMO on the other
hand is the HOMO+1. Using the Aufbau principle, we can draw up a diagram
as with Hydrogen, so we get (remember this is not starting at the 1s
orbital): Using
chemistry software it is possible to model these MOs using quantum
calculations, various types of these calculations exist with higher or
lesser degrees of accuracy. The HOMO can be seen below:
Using
chemistry software it is possible to model these MOs using quantum
calculations, various types of these calculations exist with higher or
lesser degrees of accuracy. The HOMO can be seen below:
Now let's try it with F2:
 The HOMO will be MO9, and can be represented as:
The HOMO will be MO9, and can be represented as:
Thanks for reading!
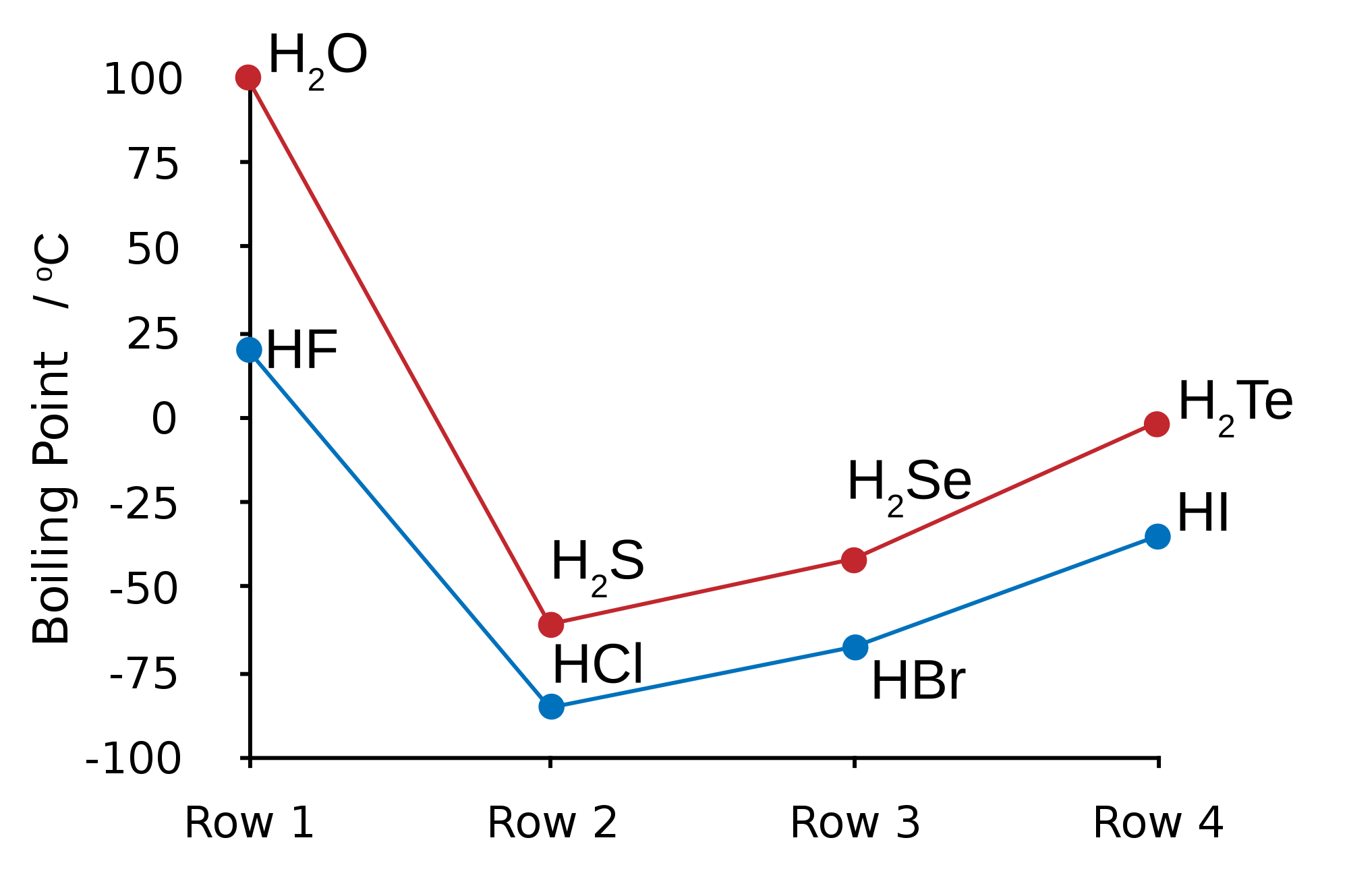
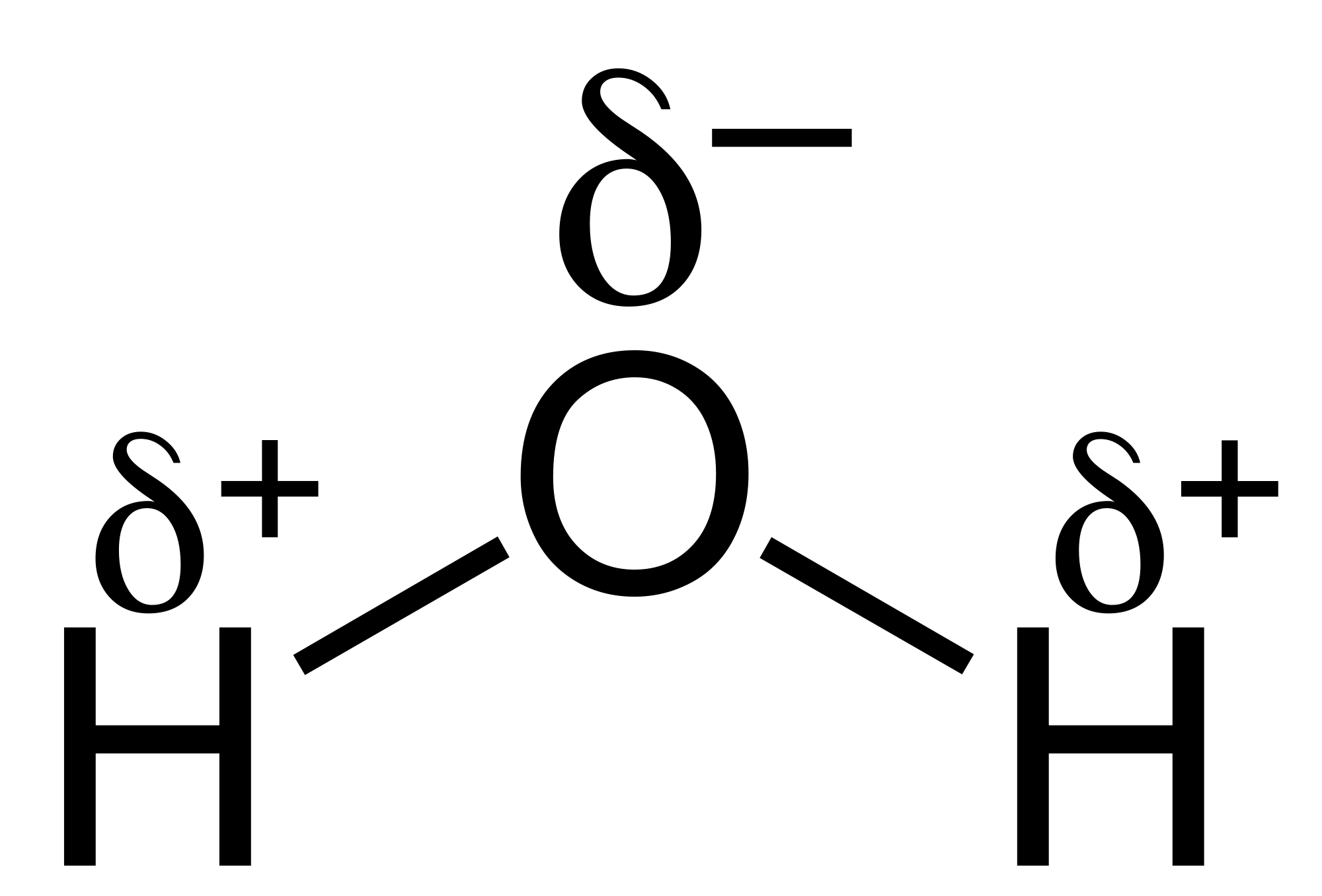
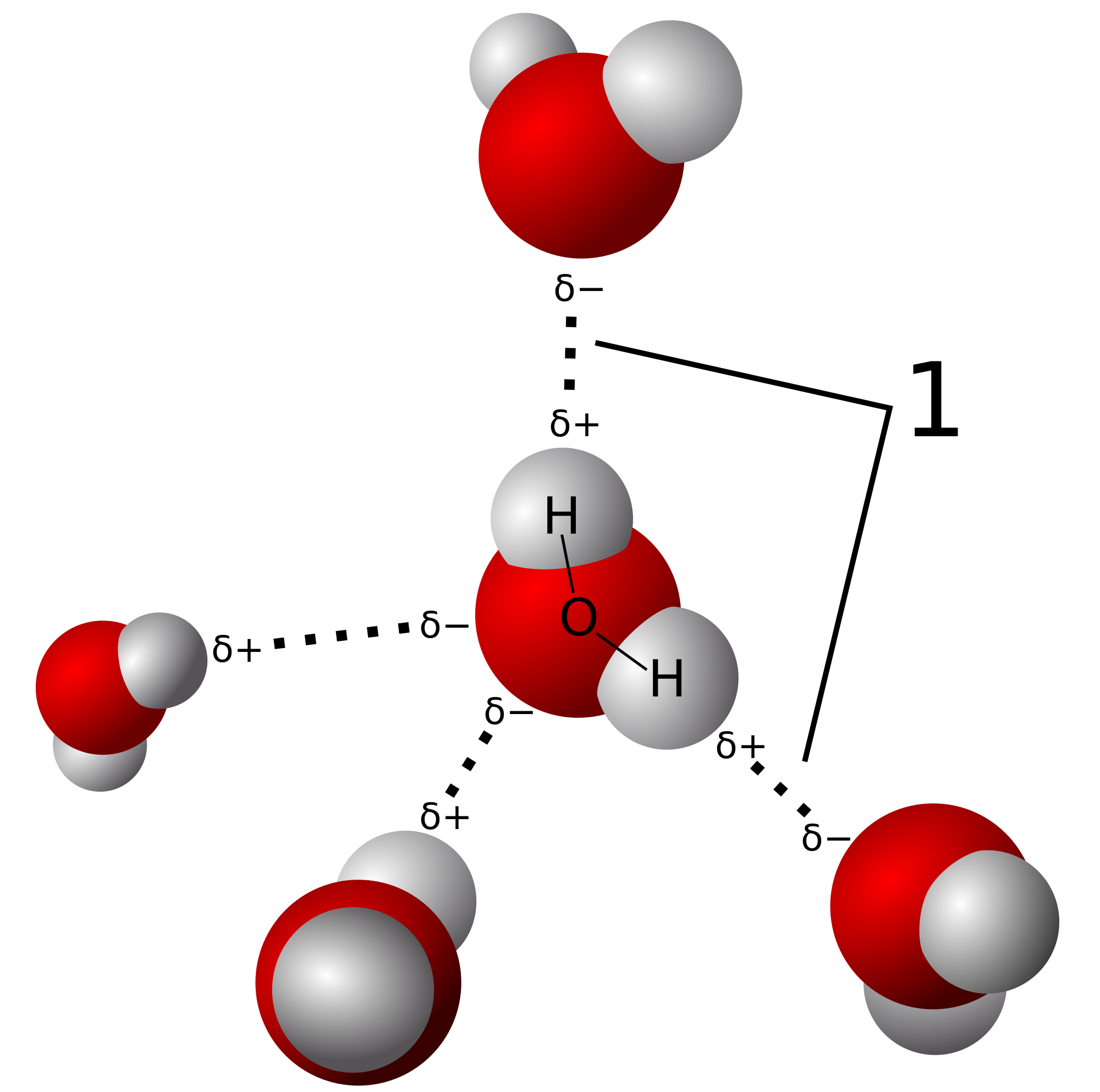
In the next part I'll go into more basic molecules of the heteronuclear diatomic type, such as hydrogen fluoride, HF.












-3D-balls.png)




