The above image is a colourised electron micrograph that of a T-cell (green) infected by HIV H9 viruses (yellow) budding on the cellular surface. It would be beautiful, were it not so deadly.
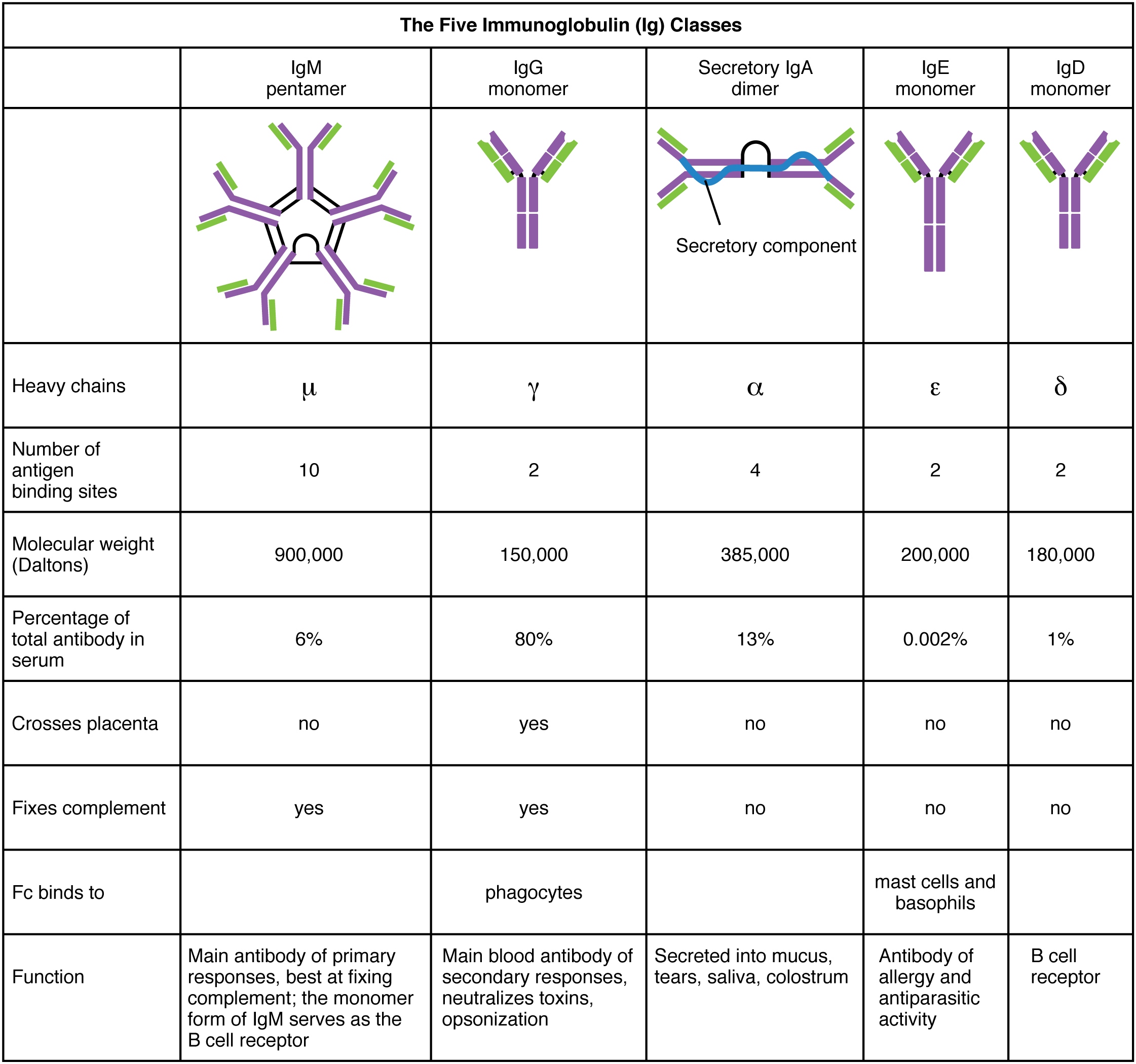
Antibodies are used in an array of different defence situations
So far, I've only covered how your immune system produces antibodies that tag and aggregate an antigen (foreign substance) which is then consumed by a macrophage (white blood cell) in a process known as the humoral immune response. But there's the other form of defense too: the cellular immune response. As we've established previously, the cellular immune response involves a specific set of cells called lymphocytes that recognise and destroy foreign cells (antigens).
When people undergo transplants, it's commonplace that a few patients' bodies will undergo tissue rejection. The cellular immune response plays a role in this phenomenon as cells that don't quite match you own have different receptors on the cell surface, this leads to lymphocytes rejecting the donor's organ as those regions are recognised as an antigen. Your body also can utilise this system to destroy any potential cancer cells before they propagate to unmanageable quantities, this doesn't always work, though.
In both humoral and cellular immune responses have a similar molecular method of recognising foreign objects by using proteins belonging to the immunoglobulin superfamily. In the four examples below, if your eyes are good, you'll notice they all share similar sheet and loop structures. PDB IDs (protein database ID) are and links to their RCSB PDB profiles are listed:


Left: A class I human major histocompatibility complex (MHC), PDB ID: 1a1m; Right: A class II human MHC, PDB ID: 1dlh


Left: A human T-cell receptor binding to a MHC class I molecule and a viral peptide; PDB ID: 1bd2; Right: A Murine Fab fragment; PDB ID: 1nca (the link shows for Fab fragments bound to a neuraminidase.
I'd definitely recommend checking out those RCSB links; they have 3D models of these molecules so you can have some idea of how they bind to antigens (some of the models include an antigen).
In the header and throughout this part I've mentioned T cells quite a lot. These cells are involved in the cellular immune response and have structures similar to the Fab fragments of antibodies on their cellular surface. Consequently, these cells target unwanted cells and are able to destroy them, which is why they have the name of Killer T cells ( or "cytotoxic T cells"). Receptors on the outside of these cells are capable of recognising foreign peptide chains on the surface of invading or infected cells. The examples shown above include MHC proteins, these are found on killer T cells and act as a switch that releases an attack protein called perforin. Perforins are released only when a T cell receptor (including the MHCs) detect an antigen, these then react with the detected invasive cell's membrane and form pores that effectively drain the cell of essential ions - killing the cell.
Why AIDS vaccines are so hard to produce based on what we've covered so far
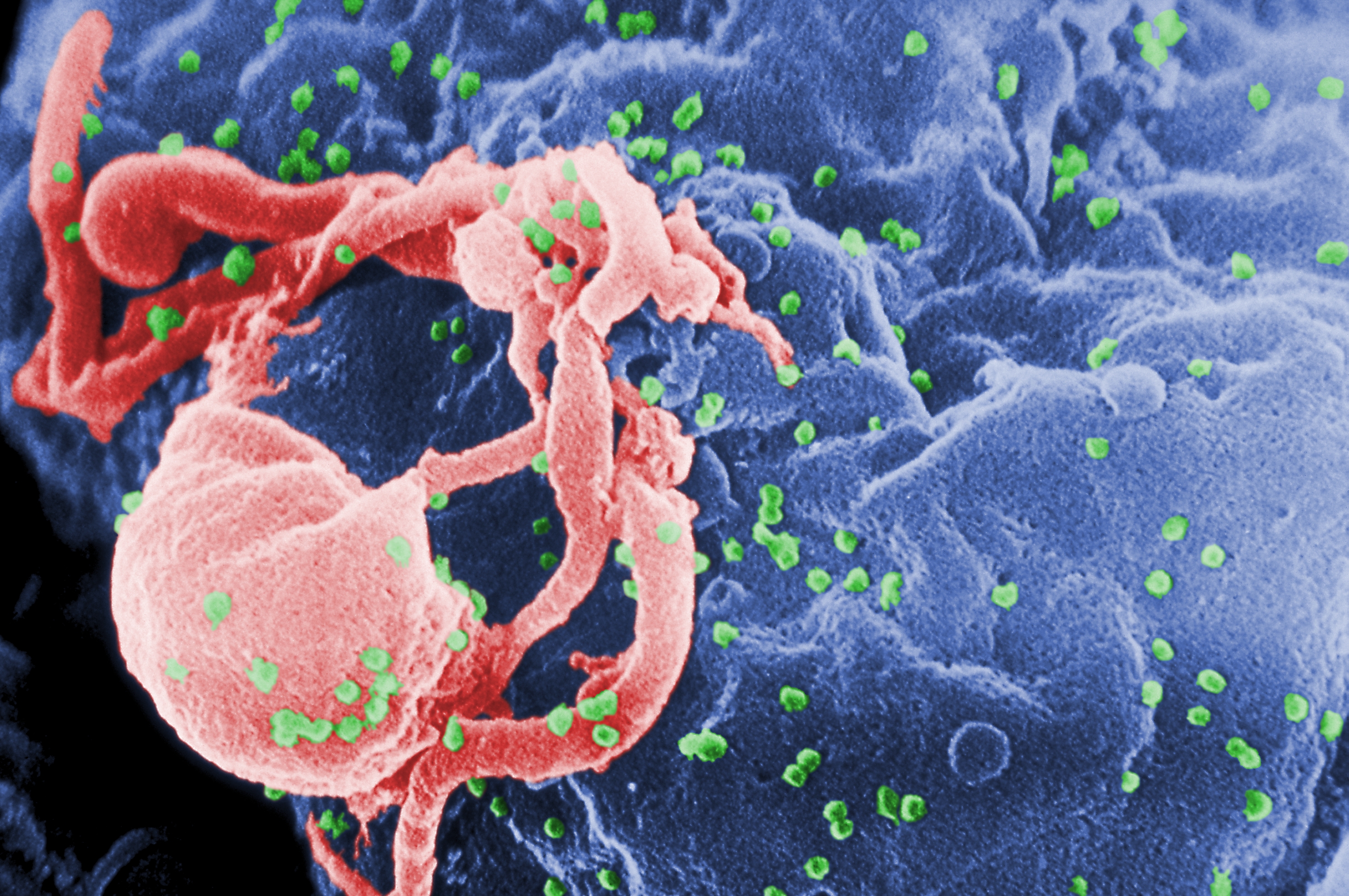
Acquired Immune deficiency syndrome (AIDS) is a symptom of the problematic human immunodeficiency virus (HIV). HIV attacks T cells that are essential in a healthy immune system. The infected type of T cells that HIV infects are part of the first line of defence against antigens - they signal a set of B cells that produce important antibodies that help identify and destroy foreign objects. In effect, HIV completely disables the body's ability to defend itself.
To make matters worse, HIV undergoes mutations in it's genome (and therefore the antigenic determinants change often) at 60 times the rate of the influenza virus. Making a flu vaccine is problematic enough because the same reason - once we make a vaccine, and many viruses are destroyed, those remaining (somewhere in the human population) have already mutated and cannot be recognised by a complementary antibody.
So far we've only been able to slow the progress of AIDS, through therapies involving drugs that target the processes that replicate the viral genome in cells.
Here's hoping some brilliant person will figure out a way to stop the AIDS pandemic which has ruined 60 million lives since 1983.
And that concludes this short three-part series! Make sure to look into more of this subject.





 A
A