Water is essential for life. Why?
Water is so normal for many of us, and we don't question why it is an essential part of life. Around 71 percent of our planet's surface is water, from the vast salt-water seas, the streams that flow from mountains and into lakes and to the frigid polar ice caps. It is also abundant in the universe, comprising of the most abundant elements: hydrogen and oxygen. It has been detected all across the milky way. In fact there is a "cloud" of water with over 140 million times the water on earth surrounding an area around a black hole called a quasar, 12 billion light years from here.
About everywhere water exists on earth, life does too. The answer lies in its chemistry - from its physical properties to how it interacts with the stuff around it
The physical properties of water
Water exists on earth in three main states: solid (ice), liquid, and gas (clouds). Water is most useful to life in liquid form at standard temperatures (25℃) and normal atmospheric pressure (1 atm). Water is also able to exist as a liquid at a broad range of temperatures, from 0℃ to 100℃ and compared to other similar compounds its boiling point is much much higher. See below:
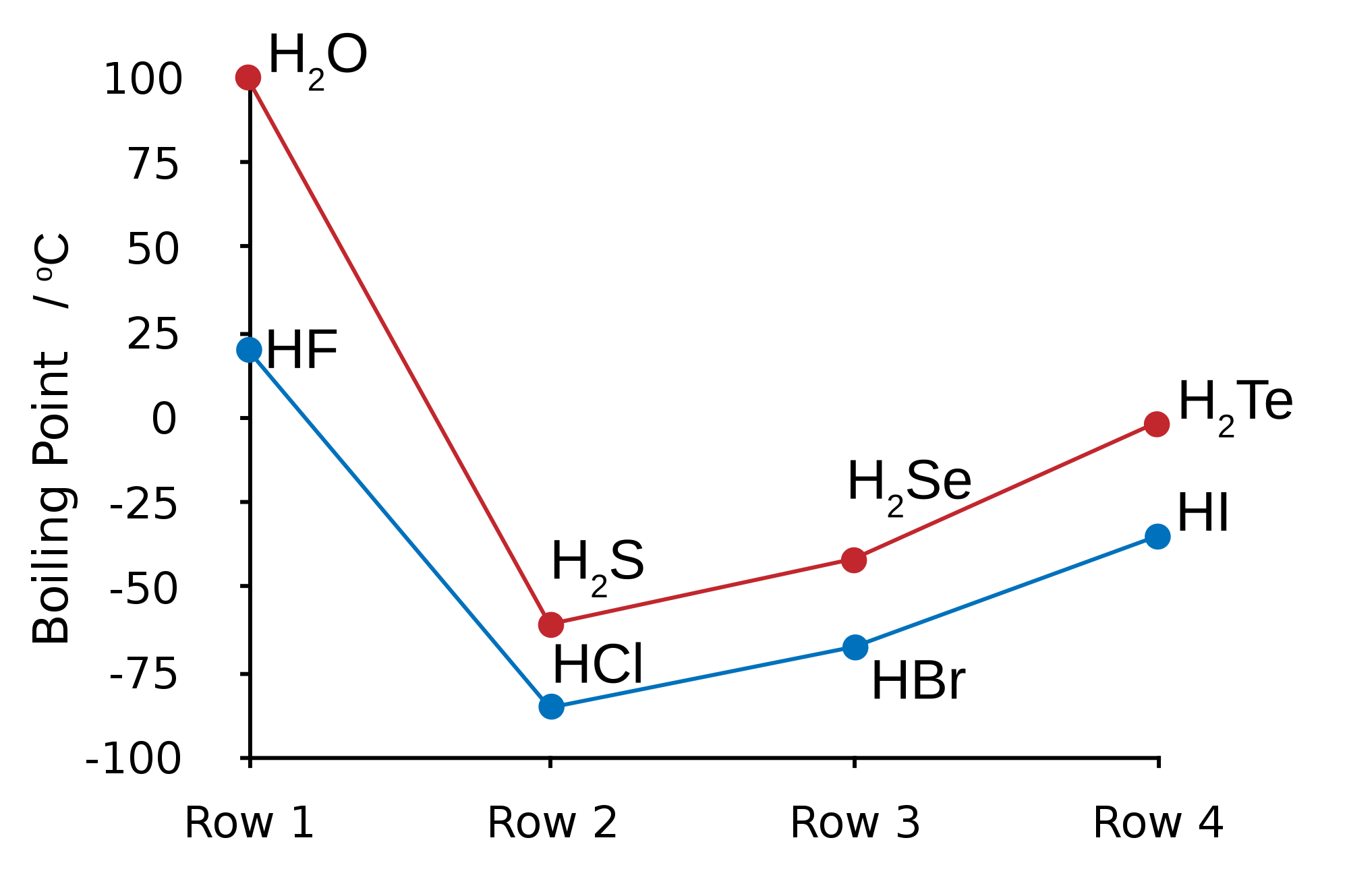
Its solid state is also different from similar compounds: it is less dense than the liquid form, which is something almost unique to water. For example, liquid water's max density is 1,000 kg/m3 and solid water has a density of 917 kg/m3. Water expands in its solid state by about 9% compared to its liquid form.
Water, Hydrogen bonding and its importance to life
Water is what we call a polar molecule, one part of the molecule is much more negatively charged than other parts of the molecule. What this forms is an electrical dipole moment, which is the separation of more negatively charged parts of a molecule, see the diagram below:
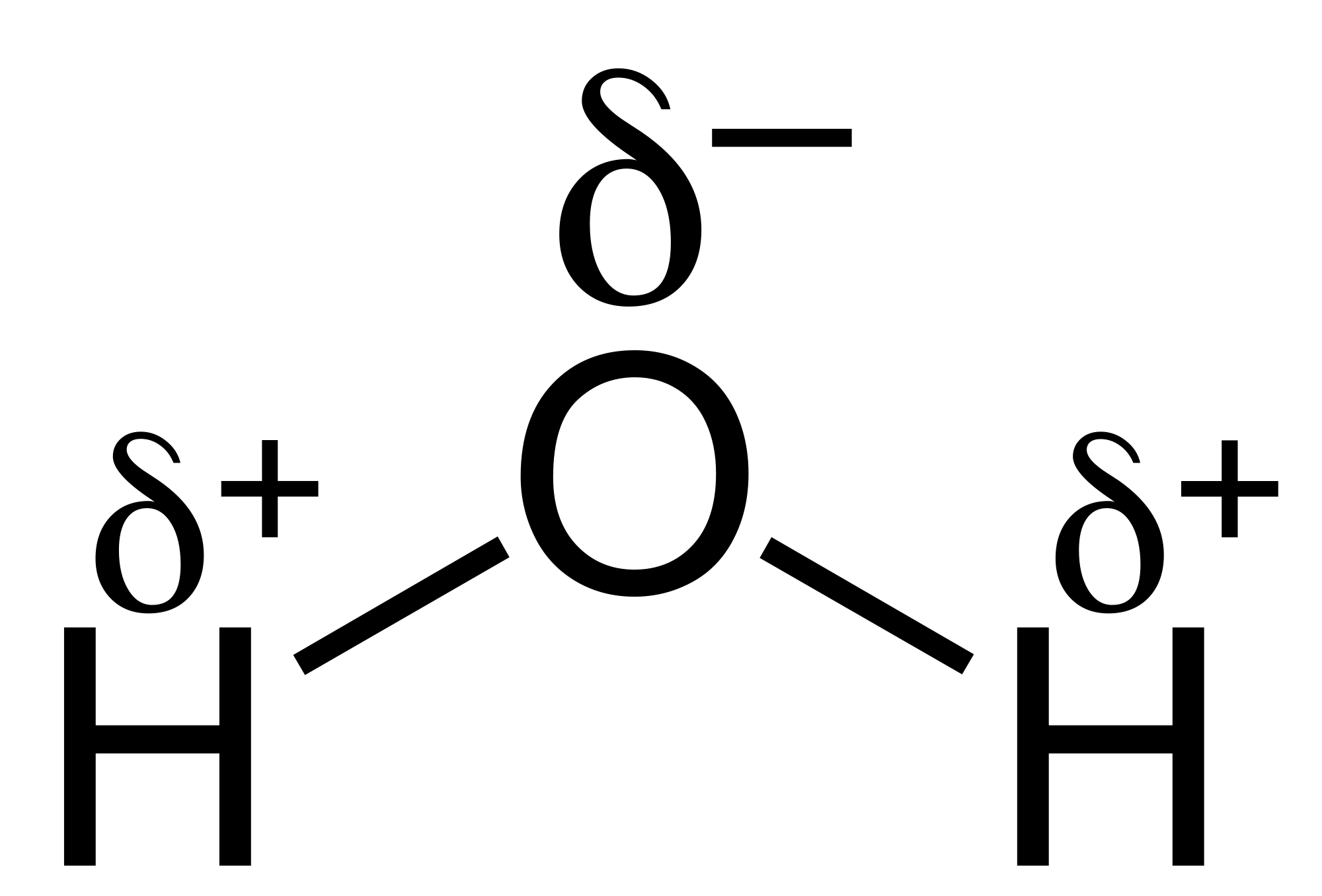
It is the separation of slight negative and positive charge that gives water its special properties. The more negative areas attract the positive areas of other molecules, when this occurs a weak bond between the water molecules form. Up to four of these "hydrogen bonds" can be formed (two on the oxygen as it has two lone pairs of electrons),
These bonds are individually quite weak and are easily broken, but in large quantities has a dramatic effect on the substance's boiling and melting points, as well as the structural properties in solid form. As discussed above, ice is more dense and expands up to 9% more than the maximum density of liquid water. This is because the orientation of hydrogen bonds in water causes the molecules in solid form to push further apart which reduces the density of the solid. Below, "1" shows where hydrogen bonds occur.
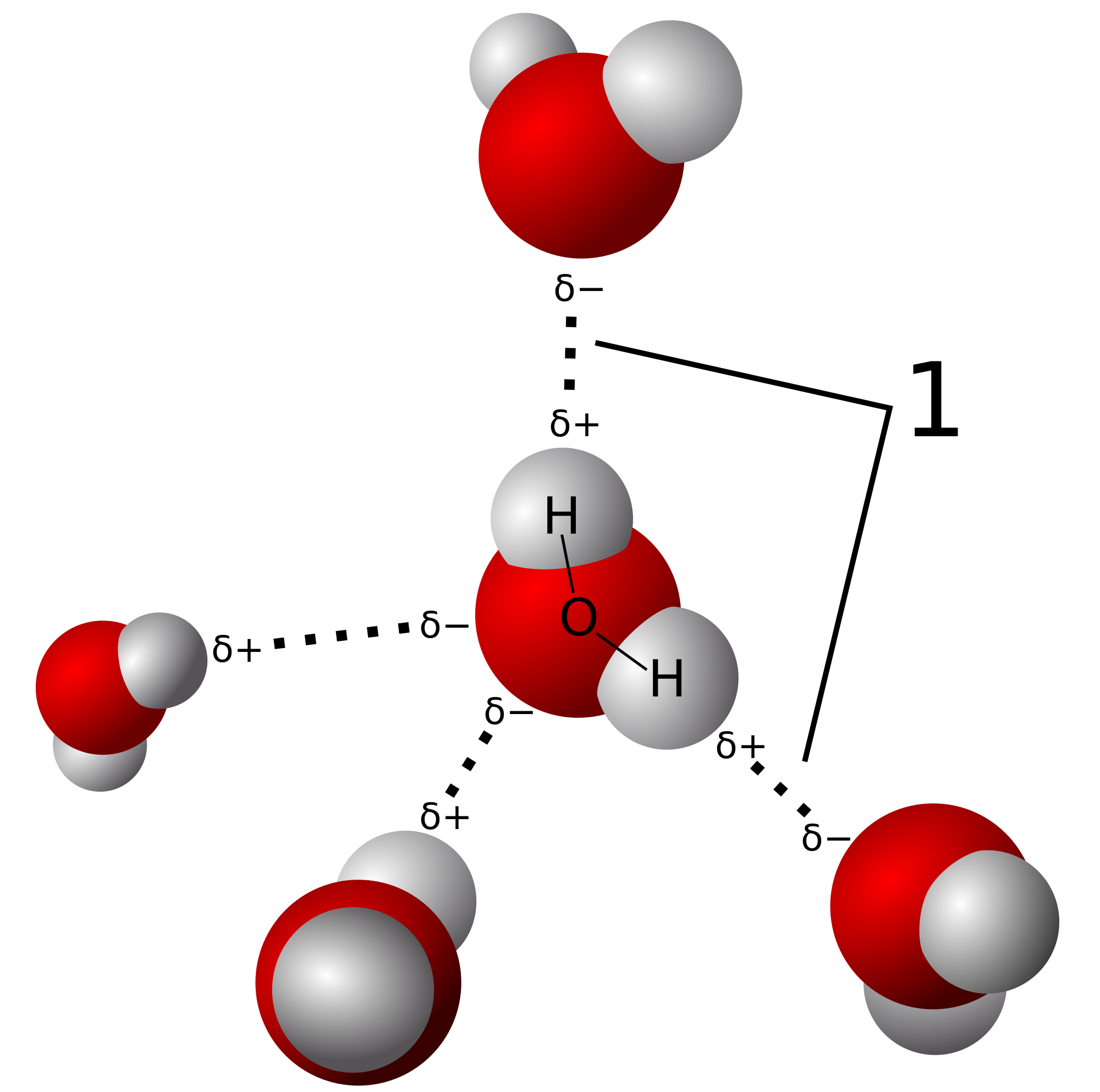
Water's polarity also enables it to dissolve other polar substances, and enables an environment for many organic compounds to react with each other, that would otherwise be impossible in solid form and quite difficult in gaseous form. Hydrogen bonding isn't just found in water present in lifeforms, but also in many organic compounds such as DNA and every protein in your body. These compounds could only exist in your body in aqueous form (mixed with water) because of water's properties as a solvent.
Water is also involved in many biological reactions in many of which occur in metabolic pathways. In anabolic pathways, water is a product of forming large and complex molecules. While in catabolic pathways, water is always one of the reactants, that "add water" to the large molecules, breaking it down.
It is also an important to the pH balance of life forms, as the hydrogen ion (H+, a proton) acts as a "Lewis acid," which donates itself to a "Lewis base," often a hydroxide ion (-OH) to form water. It is this interaction (with other acids and bases too) that has enabled us to create the pH scale, which determines the concentrations of H+ ions and -OH ions present in a solution.

The many uses of water
Water has its many uses for us humans, besides for drinking. We use it in our agricultural industries, for cleaning ourselves, moving around the world, to generate power, to extinguish fires and, to have fun and relax in. It is even used to exchange heat because of its high capacity of heat absorption. This capacity, especially for vaporization (into gaseous form), is called the enthalpy of vaporization, which is the required heat needed to convert a quantity of substance into vapor form. This capacity makes it useful in many power stations, nuclear power plants or in old machine guns (like the Vickers machine gun). Its usefulness outside of needing it to stay hydrated makes it one of the most versatile chemicals in the universe.

Water is an interesting molecule - it doesn't fit in with molecules of similar size and type, which makes it have all sorts of interesting properties not found in the same way in other molecules. It is the most important molecules that has enabled life here on earth to exist the way it does. Without it, it'd be hard to say we'd be here at all.
Don't forget to check out my Patreon, if you like the content I'm putting out:


No comments:
Post a Comment